-
-
GENE THERAPY
BIOFOUDRY™
-
Gene Therapy
Gene therapy is a next-generation modality of therapy that treats diseases by delivering genes to cells in the body. It is garnering attention as an innovative treatment for rare and intractable diseases for which no treatment has yet been established, as well as cancer and neurodegenerative diseases.
A key element in the development of gene therapy is the gene carrier (vector). In recent years, many gene therapies have utilized artificial viruses (called viral vectors) as therapeutic agents, and in particular, adeno-associated virus (AAV) vectors and lentivirus (LV) vectors have been actively used to develop gene therapy products. In addition, non-viral vectors such as lipid nanoparticles (LNPs) are widely used in mRNA drugs, which are included in gene therapy in the broad sense, to transiently express mRNA in the body.
In recent years, the development of gene therapy products has been actively promoted mainly in Europe and the United States, and the size of the global market for gene therapy products as a new therapeutic modality to save the lives of critical patients, is expected to expand rapidly in the future. On the other hand, the lack of supply of investigational new drugs needed for the development of gene therapy and soaring production costs are major challenges.
Particularly in Japan, the infrastructure for commercialization research (Chemistry, Manufacturing and Control: CMC) of gene therapy products is not well-developed, which has resulted in a bottleneck in the development of gene therapy products because investigational drugs cannot be manufactured with the quality assurance necessary to conduct clinical trials. At Synplogen, we provide a variety of services and develop technologies to rapidly bridge the gap between gene therapy product seeds and clinical trials.
Gene Therapy Biofoundry™ Services
Utilizing our proprietary DNA synthesis technologies, we have begun providing high-quality, cost-effective Gene Therapy Biofoundry™ services as a field-specific biofoundry specializing in the field of gene therapy. Specifically, we provide one-stop solution services for “sequence design and DNA synthesis,” “cell line development,” “process development (scale-up)” and “analytical development and quality control” at our functionally and physically integrated R&D center.
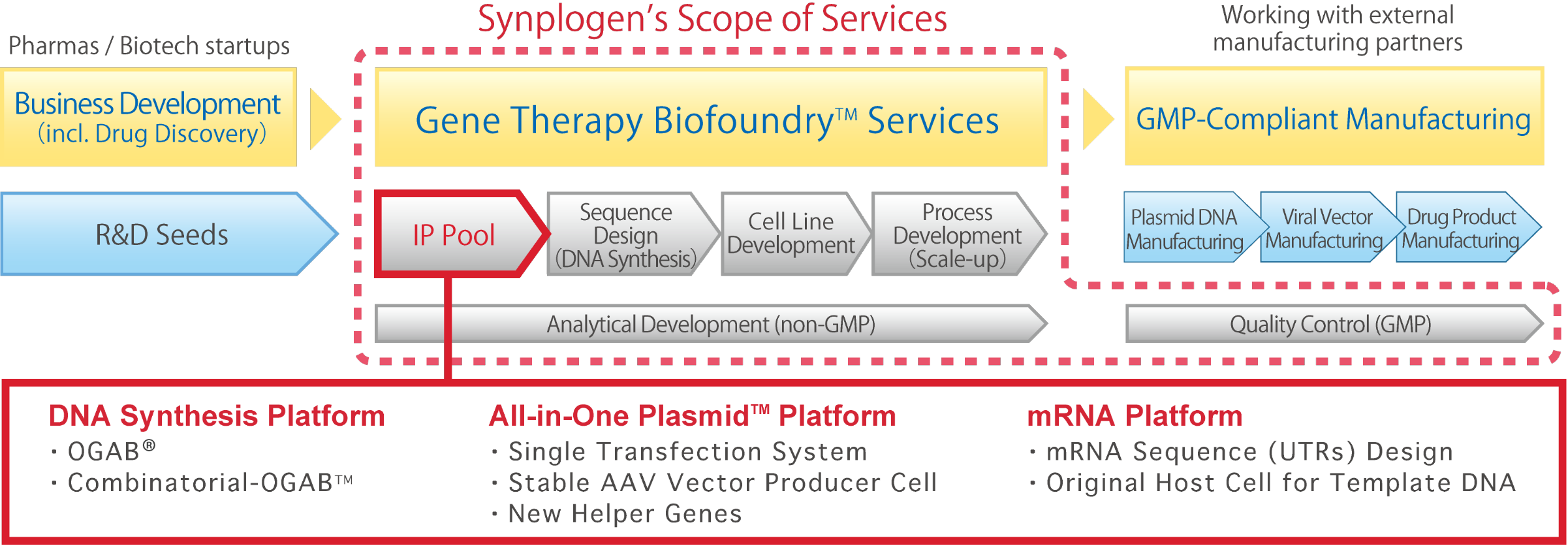
The modalities covered by our Gene Therapy Biofoundry™ services are gene therapy using viral vectors (AAV vectors or LV vectors) and mRNA medicines.
Applicable Modalities
| Modality |
|
|
|
|---|---|---|---|
| Loadable DNA Size | ~4.5 kb | ~8 kb | - |
| Expression Period |
Long Term (Non-dividing cells only) |
Long Term | Transient |
| Chromosomal Integration | Low Frequency | Yes | No |
| Typical Usage | in vivo Gene Therapy | ex vivo Gene Therapy | Vaccine / Cancer Vaccine |
We are contracted by pharmaceutical companies and drug discovery startups to develop these gene therapy products, and we, as a Gene Therapy Biofoundry™, are in charge of CMC-related tasks in the drug development process. Furthermore, by collaborating with domestic and overseas development partner companies capable of manufacturing GMP-compliant products, we have established an integrated development and manufacturing value chain, contributing to the early entry into clinical trials and early commercialization of gene therapy products that our customers aim to develop.
Synplogen's Capability
-
Sequence Design
We utilize our proprietary DNA synthesis technologies to design and synthesize DNA sequences, which are the blueprints for gene therapy products. At Synplogen, we are continuously researching and developing technologies that contribute to the design and manufacture of gene therapy products using our proprietary OGAB™ and Combinatorial-OGAB™ (Combi-OGAB™), and have them available as part of our technology lineup. We also support Feasibility Studies for the out-licensing of these technologies.

-
Process Development (scale-up)
In order to produce viral vectors and mRNA for gene therapy, plasmid DNA suitable for medical raw materials is required. At Synplogen, we develop culture processes using jar fermenters, column chromatography purification processes, etc. For viral vectors, we also develop culture processes using bioreactors, column chromatography purification processes, and pharmaceutical formulations related to the expiration date of investigational drugs.
-
Analytical Development and Quality Control
Our services range from analytical development for viral vectors and mRNA using state-of-the-art analytical instruments, to characterization and stability testing in process development, and validation in GMP-compliant analytical development. In addition, we perform quality testing of plasmid DNA, viral vectors, and formulations (final products) in GMP manufacturing, as well as GMP-compliant stability testing.
Disclaimers
- Viral vectors provided by Synplogen may only be used for research and may not be used for clinical or diagnostic applications. If the provided samples are used for anything other than research, Synplogen accepts no liability for any loss or damage that may arise from such use.
- If viral vectors are necessary for commercial use, please make this clear in your inquiry. A licensing agreement may be necessary.
- Follow all direction and guidelines for treatment and handling of viral vectors which are provided by law, ordinance (laws governing use of GMOs/LMOs for the protection of biodiversity, local regulations for the appropriate biosafety level/containment measures, etc.) or safety authority (government body or affiliated organization).

