-
-
SEQUENCE DESIGN
-
-
We utilize our proprietary DNA synthesis technology to design and synthesize DNA sequences, which are the blueprints for gene therapy products. At Synplogen, we are continuously researching and developing technologies that contribute to the design and manufacture of gene therapy products using our proprietary OGAB™ and Combinatorial-OGAB, and have them available as part of our technology lineup. We also support Feasibility Studies for the out-licensing of these technologies.
Viral Vector
Viral Vector Production Service for Research Use
We have prepared different variations of plasmid DNA (Cap, Rep, ITR, etc. derived from various AAV serotypes) for the production of AAV vectors and LV vectors. Using these plasmid DNAs for viral vectors, we provide a variety of services, from synthesis of gene sequences to production of viral vectors, to meet the needs of our clients.
- Synthesis of Gene of Interest (GOI) sequences for packaging within viral particles
- Synthesis of plasmid DNA for viral vector production
- Packaging of synthetic DNA into viral vectors
- Production of empty capsid particles
If you would like us to synthesize DNA that is to be packaged into viral particles or if you send us your pre-constructed plasmid DNA, we will deliver the viral vectors with the desired GOI within approximately one month after obtaining the DNA. Genome titer quantitative analysis is performed as standard, and various quality tests are also available as options. Please provide us with the following information when you use our packaging services.
- AAV serotypes (e.g., 1, 2, 5, 6, 8, 9)
- Desired amount of viral vector to be obtained (e.g., 1.0E+13 vector genome)
- Number of density gradient ultracentrifugation (e.g., once or twice)
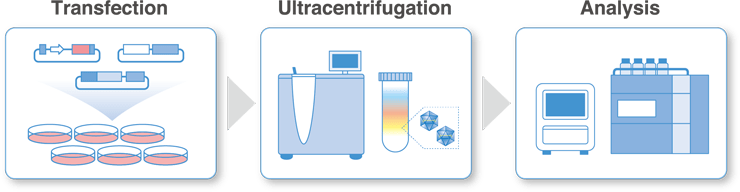
We offer production and analysis services in the BSL-2 viral vector production area.

Technology Lineup
-
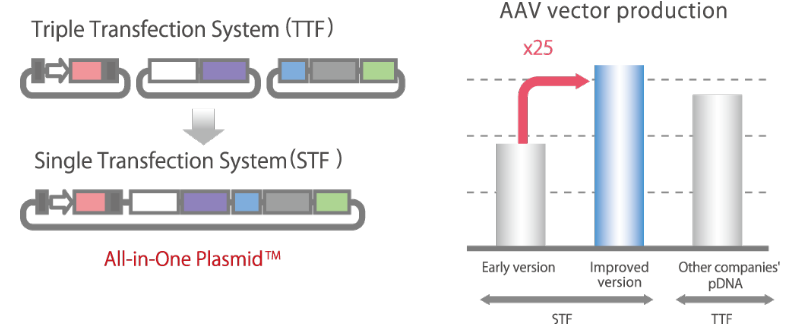
Significant Reduction in Viral Vector Production Costs:
All-in-One Plasmid™Currently, the mainstream method for producing AAV vectors for gene therapy is to use three types of plasmid DNA, but with the All-in-One Plasmid™ developed by Synplogen, all the genes necessary for the production of AAV vectors can be carried in a single plasmid. Our All-in-one Plasmid™ technology significantly reduces the cost of producing AAV vectors.
-
-
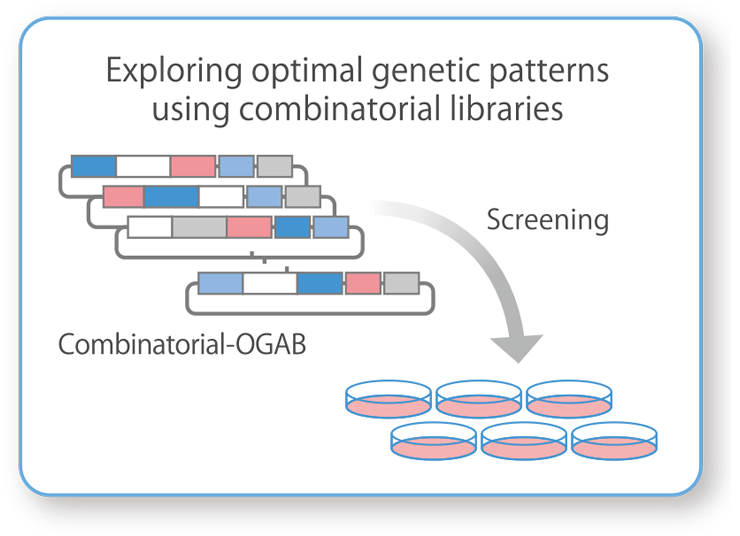
Exploration of Genes That Increase the Production of Viral Vectors:
Combinatorial-OGABCombinatorial-OGAB is used to design and construct large and diverse DNA libraries. We can quickly search for the best gene clusters to meet the client's needs, such as increasing the production of viral vectors.
-
mRNA Medicines
We have technologies related to sequence design that contribute to the efficacy of mRNA medicines and together with our proprietary DNA synthesis technology, we can provide a comprehensive service from mRNA design to synthesis of template DNA and target mRNA.
Technology Lineup
-
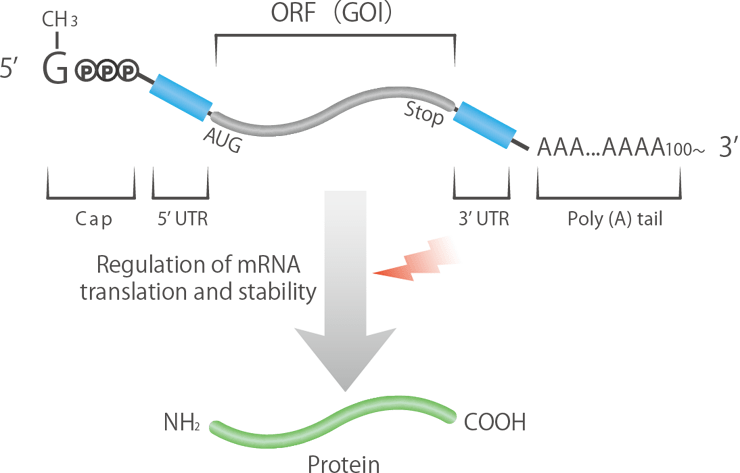
Exploration of mRNA Sequences That Improve Protein Expression
The expression efficiency and stability of mRNA medicines depend largely on the sequence design of each region that makes up the mRNA. We design and synthesize unique mRNA sequences, including untranslated regions and poly-A strands, by leveraging the strengths of our proprietary DNA synthesis technology.
-
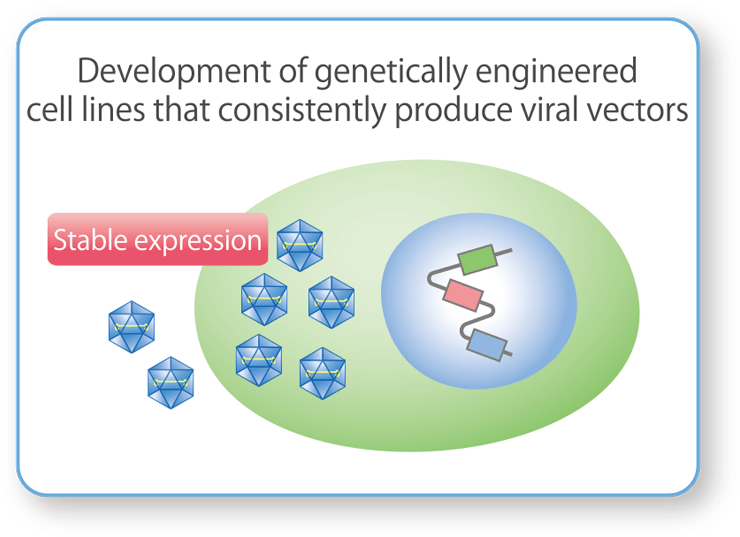
Cell Line Development
We develop unique host cells that produce the target production substances (plasmid DNA and viral vectors) to improve their yield and purity. The main target cells for plasmid DNA are E. coli, and for viral vectors are genetically engineered cell lines (HEK293 cells).
Technology Lineup
-
Host Cell Development for Stable Production of AAV Vectors
We develop HEK293 cells that stably produce AAV vectors by incorporating unique DNA constructs into host cells that allow for the regulation of toxic gene expression.
-
CONTACT
If you have any questions about our services and technologies,
or if you would like to discuss collaboration, media coverage,
etc., please contact us using the form below.
Disclaimers
- Viral vectors provided by Synplogen may only be used for research and may not be used for clinical or diagnostic applications. If the provided samples are used for anything other than research, Synplogen accepts no liability for any loss or damage that may arise from such use.
- If viral vectors are necessary for commercial use, please make this clear in your inquiry. A licensing agreement may be necessary.
- Follow all direction and guidelines for treatment and handling of viral vectors which are provided by law, ordinance (laws governing use of GMOs/LMOs for the protection of biodiversity, local regulations for the appropriate biosafety level/containment measures, etc.) or safety authority (government body or affiliated organization).

