-
-
ANALYTICAL DEVELOPMENT
& QUALITY CONTROL
-
Analytical Development
Our services range from analytical development for viral vectors and mRNA using state-of-the-art analytical instruments, to characterization and stability testing in process development, and validation in GMP-compliant test method development.
-
Cell-based assay is also available as a test method. We will discuss with you the appropriate assay system, taking into account the Mode of Action (MoA) of the gene therapy product you are developing, and propose a test plan up to GMP manufacturing.
-

Cell-based Assay
-
We will develop cell-based assays to ensure the potency of gene therapy products in specification tests, etc. In the case of gene therapy, the studies related to titer (potency) vary depending on the target of measurement, and are assumed to quantify (1) infected viral vectors, (2) transcribed mRNA, (3) translated protein, (4) translated protein activity, etc.
-
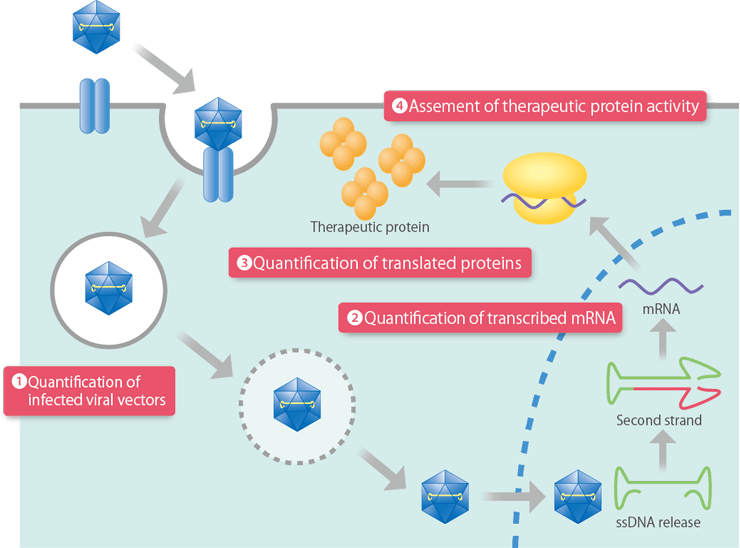
Quality Control
We conduct quality testing of plasmid DNA, viral vectors, and formulations (final products) in GMP manufacturing, as well as GMP-compliant stability testing.
Analytical Equipment
-
-
Digital PCR
GMP -
Example of tests:
Genome titer, nucleic acid-related impurities, cell-based assay
-
-

-
Gel Imager
GMP -
Example of tests:
Western blotting, SDS-PAGE
-
-

-
Next Generation Sequencer
-
Example of tests:
Nucleic acid sequence integrity, nucleic acid-related impurity analysis
-
-

-
Capillary Electrophoresis
-
Example of tests:
Purity of ccc DNA, VP1/VP2/VP3 ratio of AAV, mRNA sequence integrity
-
-

-
Multi-angle Dynamic Light Scattering
-
Example of tests:
Particle size distribution, particle concentration
-
-

-
Quaternary UHPLC
GMP -
Example of tests:
Empty capsid ratio, purity of ccc DNA, various residual impurities
-
-

-
Plate Reader
GMP -
Example of tests:
Viral particle titers (ELISA), host cell protein
-
-

-
Flow Imaging
-
Example of tests:
Micro size particle concentration (aggregation, etc.), insoluble particulates
-
-

-
Mass Photometry
-
Example of tests:
Particle concentration, molecular weight, empty capsid ratio
-
-

-
Bioanalyzer
GMP -
Example of tests:
Nucleic acid and protein size
-
-

-
Thermal Stability Analysis
-
Example of tests:
Melting temperature (Tm), aggregation temperature (Tagg)
-
-

-
Cell Sorter
-
Example of tests:
Cell-based Assay
-
CONTACT
If you have any questions about our services and technologies,
or if you would like to discuss collaboration, media coverage,
etc., please contact us using the form below.
Disclaimers
- Viral vectors provided by Synplogen may only be used for research and may not be used for clinical or diagnostic applications. If the provided samples are used for anything other than research, Synplogen accepts no liability for any loss or damage that may arise from such use.
- If viral vectors are necessary for commercial use, please make this clear in your inquiry. A licensing agreement may be necessary.
- Follow all direction and guidelines for treatment and handling of viral vectors which are provided by law, ordinance (laws governing use of GMOs/LMOs for the protection of biodiversity, local regulations for the appropriate biosafety level/containment measures, etc.) or safety authority (government body or affiliated organization).

