-
-
PROCESS
DEVELOPMENT
-
Plasmid DNA
In order to produce viral vectors and mRNA for gene therapy, plasmid DNA suitable for medical raw materials is required. At Synplogen, we will study the following steps to develop a culture process using jar fermenters, a column chromatography purification process, etc. In addition, we will transfer the technology of the process we have developed for the production of plasmid DNA for GMP raw materials.
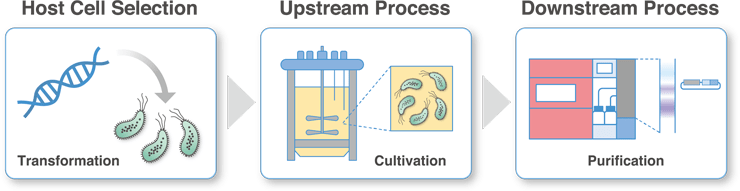
Host Cell Selection
The production of viral vectors requires large quantities of high quality plasmid DNA as a pharmaceutical raw material. Before starting the study of mass culture conditions, we select a highly productive E. coli strain that can stably retain DNA for the plasmid DNA to be used in development.
Upstream Process Development
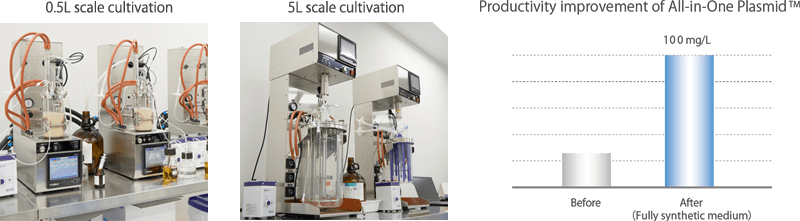
Culture conditions for the selected E. coli strains will be studied using jar fermenters. After optimizing the culture conditions in a small-scale culture, scale-up will be performed using chemical engineering methods.
- Our Equipment:
- Jar fermenters: 1L, 3.75L (single-use), 10L, etc.
Downstream Process Development
After culturing, the bacteria will contain many impurities (host-derived proteins, endotoxins, linear raw material DNA, etc.). We will develop purification processes using hollow fiber filters and columns in anticipation of technology transfer to GMP raw material manufacturing facilities.
- Our Equipment:
- AKTA avant 150, Krosflo® KR2i, etc.

Technology Transfer
We will transfer the technology for the manufacturing process of plasmid DNA developed by Synplogen to our GMP raw material manufacturing partner companies (or manufacturing facilities owned by the client, etc.).
- A case study of our collaboration with MicroBiopharm Japan Co., Ltd.:
- Click here for more information.
Viral Vector
In the following steps, we will develop the culture process using bioreactors for viral vectors, the column chromatography purification process, and the pharmaceutical formulation related to the expiration date of the investigational drug, and transfer the technology for GMP manufacturing of the investigational drug for gene therapy clinical trials. Adopting the concept of Quality by Design, we design manufacturing processes based on scientific validity while utilizing the latest digital technologies.
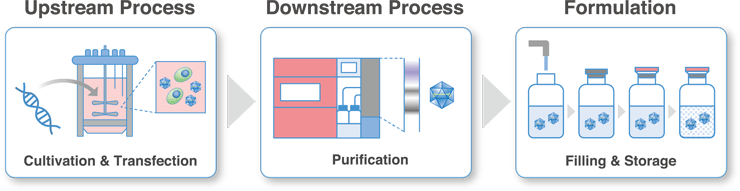
Upstream Process Development
We have both adhesive and suspension culture platforms using bioreactors in anticipation of technology transfer for the manufacturing of investigational drugs. Viral vector productivity can be maximized while monitoring the culture medium environment with various sensors. As host cells, we can use HEK293 cells that have been deposited into the cell bank by us or cells that have already been deposited by the client. In addition to the globally used triple transfection method, we also support process development using our proprietary All-in-One Plasmid™.
- Our Equipment
-
Fixed-bed reactor: Scale-X hydro(2.4m2)
Stirred tank reactor: 0.04L, 2L, 2.5L (single-use), 10L (single-use), 50L (single-use, will be ready by the end of 2023)
Culture medium analysis: Vi-CELL MetaFLEX, etc.
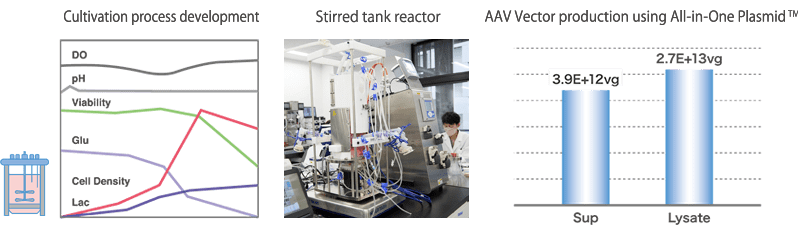
In order to practice scale-up based on scientific validity, we are building a Scale Down Model (SDM) for viral vector culture production. First, the process is optimized by Design of Experiment (DOE) and other methods using SDM, and then scale-up studies are conducted using chemical engineering methods (kLa, P/V, etc.) and simulation techniques.
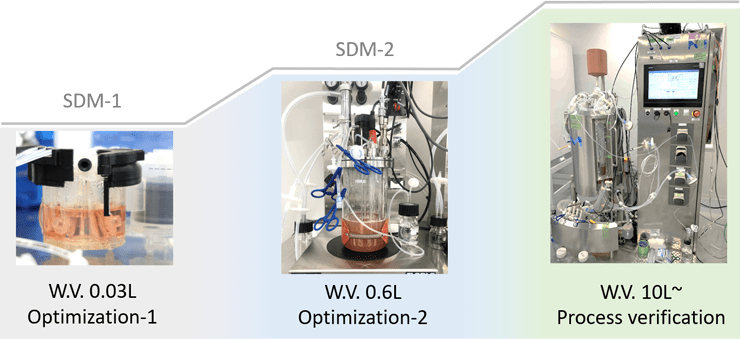
Downstream Process Development
After incubation, the harvest solution contains many impurities (e.g., empty capsid particles, host-derived proteins, raw DNA, etc.). We will develop purification processes using hollow fiber filters and columns in anticipation of technology transfer to GMP manufacturing facilities.
- Our Equipment:
- AKTA avant 150、KrosFlo® KR2i, etc.

Formulation Development
Using the viral vectors obtained in the process development study, we will conduct stability evaluation using our standard formulation and obtain data on the basis for setting the expiration date of the investigational drug.
- Examples of stability test endpoints:
- genome titer, TCID50, pH, insoluble particulates, insoluble foreign matter, properties, etc.
Technology Transfer
We will transfer the technology for the manufacturing process of the viral vectors developed by Synplogen to our GMP manufacturing partner companies (or manufacturing facilities owned by the client, etc.).
- A cases study of our collaboration with Merck:
- Click here for more information
CONTACT
If you have any questions about our services and technologies,
or if you would like to discuss collaboration, media coverage,
etc., please contact us using the form below.
Disclaimers
- Viral vectors provided by Synplogen may only be used for research and may not be used for clinical or diagnostic applications. If the provided samples are used for anything other than research, Synplogen accepts no liability for any loss or damage that may arise from such use.
- If viral vectors are necessary for commercial use, please make this clear in your inquiry. A licensing agreement may be necessary.
- Follow all direction and guidelines for treatment and handling of viral vectors which are provided by law, ordinance (laws governing use of GMOs/LMOs for the protection of biodiversity, local regulations for the appropriate biosafety level/containment measures, etc.) or safety authority (government body or affiliated organization).

